GSK US Independent Medical Education
Closing the gaps and improving patient health
As part of GSK's mission to get ahead of disease together, GSK identifies and funds innovative, high-quality, independent third-party educational initiatives that are designed to close US healthcare professional (HCP) educational, quality, and performance gaps - with the ultimate goals to reduce healthcare disparities, improve patient health, and enhance patient quality of life.
Independent Medical Education (IME) for HCPs
GSK provides financial support for IME for HCPs, including certified continuing medical education (CME, CE).
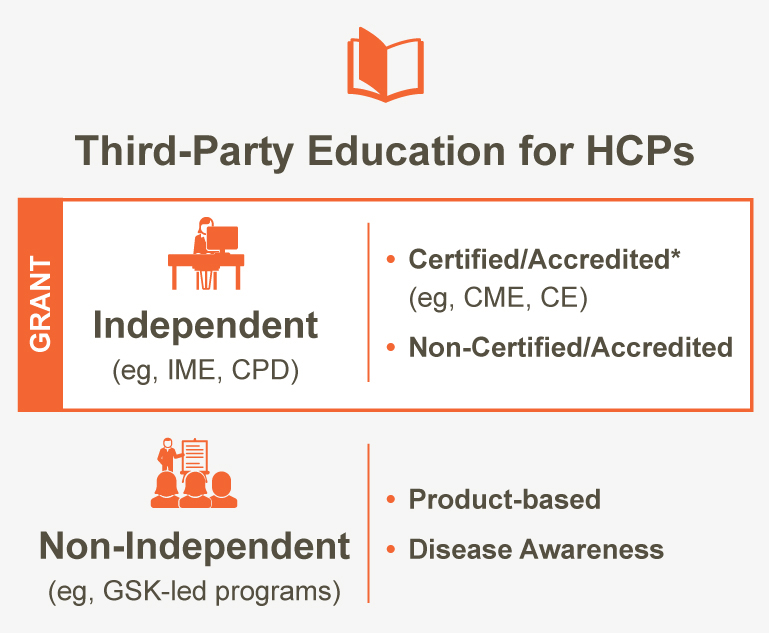
GSK defines IME for HCPs as any third-party education for HCPs in which GSK (or any commercial interest) has no control, influence, or involvement (ie, independent).
At GSK, IME (certified and non-certified) is funded with a grant.
What is the difference between IME and certified continuing medical education (CME)?
- Certified CME is a type of IME. Not all IME is certified CME; however, all certified CME is IME.
- If the IME is certified/accredited, it is referred to as certified CME. Continuing education (CME or CE) credits are offered for HCP participation.
- IME may also be non-certified/non-accredited. In this case, no CME or CE credits are offered for HCP participation.
*CME/CE credits for HCPs
HCP: Healthcare professional
IME: Independent medical education
CME: Certified continuing medical education
CE: Certified continuing education
CPD: Continuing professional development
Eligibility
There is a 2-step process for US-based organizations to submit US IME grants to GSK:
Organizations must first register and demonstrate that they are an eligible educational provider.
Eligible educational providers may submit grants in response to the US Call for Grant Applications.
Educational providers must meet the below eligibility criterion:
Accredited to provide HCP continuing education (ie, CME, CE) by a US national accrediting body such as the Accreditation Council for Continuing Medical Education (ACCME), Accreditation Council for Pharmacy Education (ACPE), American Nurses Credentialing Center (ANCC), or American Association of Nurse Practitioners (AANP)
Call for Grant Applications (CGA)
Grant proposals will be evaluated for compliance and merit using the disease areas of interest and grant review criteria detailed in the US Call for Grant Applications.
Funding priorities will focus on independent educational initiatives designed to measure improvements/changes in the following:
1Moore DE, et al. J Contin Educ Health Prof. 2009;29:1-15.
Grant requests will be prioritized based on optimal alignment with:
Please review the US Call for Grant Applications for more details about the grant submission dates, disease areas of interest, and grant review criteria we use to evaluate grants submitted for compliance and merit.
Ready to request funding?
Your first step will depend on whether you already have an account in GSK's Grant Management System. Get started by selecting the appropriate option below.
If you have a user account, you can log in with your username and password and submit a funding request.
Log In NowIf you do not have a user account, please follow these steps:
Visit the GSK Grant Management System and click the "I'm Ready to Register" button.
Enter your information on the registration form and click "Register" to proceed to the next step.
If you have already registered your account but have trouble logging in:
Click "Forgot Password?" to reset your password for access to the system. If you don't receive the email to reset your password in your Inbox, please check your Spam/Junk email folder.
